Mastering Chemistry Student Guide
Buy Mastering Chemistry Student Access Kit on Amazon.com FREE SHIPPING on qualified orders. Student Support. Still need help? Sign-In Help; Student User Guide; Contact Pearson Support; Accessibility Information; Site Map. Mastering Chemistry Sign In. Study 27 Mastering Chemistry Chapter 3 flashcards from Shaun B. On StudyBlue.
This work is protected by local and international copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permitted. The work and materials from this site should never be made available to students except by instructors using the accompanying text in their classes. All recipients of this work are expected to abide by these restrictions and to honor the intended pedagogical purposes and the needs of other instructors who rely on these materials.
For courses in Organic Chemistry (2-Semester) This package includes MasteringChemistry ™. A framework for organic chemistry built around the similarities in reaction types Paula Bruice’s presentation in Organic Chemistry, Eighth Edition provides mixed-science majors with the conceptual foundations, chemical logic, and problem-solving skills they need to reason their way to solutions for diverse problems in synthetic organic chemistry, biochemistry, and medicine. The Eighth Edition builds a strong framework for thinking about organic chemistry by unifying principles of reactivity that students will apply throughout the course, discouraging memorization. With more applications than any other textbook, Dr. Bruice consistently relates structure and reactivity to what occurs in our own cells and reinforces the fundamental reason for all chemical reactions—electrophiles react with nucleophiles.
New streamlined coverage of substitution and elimination, updated problem-solving strategies, synthesis skill-building applications and tutorials guide students throughout fundamental and complex content in both the first and second semesters of the course. Personalize learning with MasteringChemistry. MasteringChemistry from Pearson is the leading online homework, tutorial, and assessment system, designed to improve results by engaging students before, during, and after class with powerful content. Instructors ensure students arrive ready to learn by assigning educationally effective content before class, and encourage critical thinking and retention with in-class resources such as Learning Catalytics ™. Students can further master concepts after class through traditional and adaptive homework assignments that provide hints and answer-specific feedback. The Mastering gradebook records scores for all automatically graded assignments in one place, while diagnostic tools give instructors access to rich data to assess student understanding and misconceptions.
Mastering brings learning full circle by continuously adapting to each student and making learning more personal than ever—before, during, and after class. Features Personalize learning with MasteringChemistry™. MasteringChemistry from Pearson is the leading online homework, tutorial, and assessment system, designed to improve results by engaging students before, during, and after class with powerful content. Instructors ensure students arrive ready to learn by assigning educationally effective content before class, and encourage critical thinking and retention with in-class resources such as Learning Catalytics ™.
Students can further master concepts after class through traditional and adaptive homework assignments that provide hints and answer-specific feedback. The Mastering gradebook records scores for all automatically graded assignments in one place, while diagnostic tools give instructors access to rich data to assess student understanding and misconceptions.
Mastering brings learning full circle by continuously adapting to each student and making learning more personal than ever—before, during, and after class. Before Class. NEW! Organic Chemistry Dynamic Study Modules focus on general chemistry remediation, acid-base chemistry, functional groups, nomenclature, and key mechanisms. The modules help students study effectively on their own by continuously assessing their activity and performance in real time. Here's how it works: students answer a set of questions and indicate their confidence level for each answer.
The questions repeat until students answer them all correctly and confidently. Once completed, the module explains the key concept. Dynamic Study Modules are available as graded assignments for prior to class, and are accessible on smartphones, tablets, and computers.

Pearson eText 2.0 features include:. Available on smartphones and tablets.
Seamlessly integrated videos and other rich media. Accessible (screen-reader ready). Configurable reading settings, including resizable type and night reading mode. Instructor and student note taking, highlighting, bookmarking, and search During Class.
NEW! Learning Catalytics ™generates class discussion, guides your lecture, and promotes peer-to-peer learning with real-time analytics. MasteringChemistry with eText now provides Learning Catalytics—an interactive student response tool that uses students’ smartphones, tablets, or laptops to engage them in more sophisticated tasks and thinking. Instructors can:. Pose a variety of open-ended questions that help students develop critical thinking skills around structure and reactivity in organic chemistry with 10 new Learning Catalytics questions for each chapter of Organic Chemistry, Eighth Edition.
Monitor responses to find out where students are struggling. Use real-time data to adjust the instructional strategy and engage students during class. Manage student interactions by automatically grouping students for discussion, teamwork, and peer-to-peer learning After Class. NEW!
MasteringChemistry's Organic Chemistry Drawing Tool is a customized version of Java Free MarvinSketch that accommodates the diversity of structures and reaction mechanisms inherent to learning organic chemistry while providing students with a wrong answer specific feedback. This educational version of MarvinSketch has been customized in response to input from hundreds of undergraduate students. The drawing tool includes comprehensive MasteringChemistry tutorials, specific to drawing with MarvinSketch, that equip students to start quickly drawing organic structures and mechanisms to complete homework. The tutorials cover how to accurately draw reaction mechanisms, how to modify answers, and how to use the palette. All mechanism-based problems provide feedback specific to each step of the reaction, and new visual cues help clarify exact placement of arrows, enable selection of the electron, and highlight which bonds have been formed or broken. Assignable, textbook specific skill building tutorials guide students through the toughest topics in organic chemistry including:.
Acids and Bases (after chapter 2). Using Molecular Models (after Chapter 3). Interconverting Structural Representations (after Chapter 4). Drawing Curved Arrows (after Chapter 5). Drawing Resonance Contributors (after chapter 8).
Drawing Curved Arrows in Radical Systems (after Chapter 13). Synthesis and Retrosynthetic Analysis (after Chapter 17).
Rate Changes and Kinetics (Appendix II). NEW! Six NMR/IR Spectroscopy simulations (a partnership with ACD labs) allow professors and students access to limitless spectral analysis with guided activities that can be used in the lab, in the classroom, or after class to study and explore spectra virtually. Activities authored by Mike Huggins, University of West Florida, prompt students to utilize the spectral simulator and walk them through different analyses and possible conclusions. 120 Organic Chemistry-specific tutorials built around the most challenging topics in Organic Chemistry provide wrong answer specific feedback and Socratic and declarative hints so students learn where they are going wrong. 1500 automatically graded questions can be assigned as homework or practice. These questions are specific to the Eighth Edition and the majority requires drawing structures and reactions.
Enhanced end-of-chapter problems now includewrong-answer specific feedback on all mechanism problems so students have the opportunity to practice and test their understanding of organic reactivity outside of class with the help and ease of an updated drawing tool and detailed feedback on their work. About the Book A modern, streamlined organization emphasizes unifying principles of reactivity, offering an economy of presentation and discouraging memorization.
The text consistently highlights the fundamental reason for all chemical reactions — electrophiles combine with nucleophiles – in order to keep students focused on the key ideas. Students are introduced to synthetic and retrosynthetic chemistry early on, allowing them to grasp multistep synthesis from the beginning. The textbook bridges the gap between organic chemistry and biochemistry Because bioorganic chemistry is the bridge between organic chemistry and biochemistry, the text emphasizes thatthe organic reactions that chemists carry out in the laboratory are similar to those performed by nature inside a cell.
These connections are especially important to biological science majors. In Chapters 1-20, the bioorganic material is presented as “interest boxes” and within the last sections of the chapters so that this material is available to the student without requiring the instructor to introduce bioorganic topics into the course. Chapters 21–26 focus on the organic chemistry of living systems. These chapters have the unique distinction of containing more chemistry than is typically found in the corresponding parts of a biochemistry text.
Chapter 21 Amino Acids, Peptides, And Proteins. Chapter 22 Catalysis In Organic Reactions And In Enzymatic. Chapter 23 The Organic Mechanisms Of The Coenzymes, Compounds Derived From Vitamins. Chapter 24 The Organic Chemistry Of The Metabolic Pathways. Chapter 25 The Organic Chemistry Of Lipids.
Chapter 26 The Chemistry Of The Nucleic Acids UPDATED! Revised, accuracy-checked text provides increased exam relevancy.
To better prepare students for the MCAT exam, MCAT learning outcomes and MCAT-style questions have been added to the Student’s Study Guide and Solutions Manual, as well as MasteringChemistry. Contributing author Richard Morrison, University of Georgia, has reviewed and honed the problem solving presentations, end of chapter problems, and the solutions manual. Improved visuals and organization engage students with difficult subject matter, organizes the chapter content and improves ease of use:.
NEW! Expanded annotations reinforce the revised art program and help keep students focused on the most important material. Numerous subheads are part of a redesigned text that enhances ease of use and gives the text a modern look and feel. Subheads help students locate important topics, show how that content develops within the section, and break the presentation into “bite-sized” portions that are easier to comprehend and connect. Strengthened emphasis on the strategies needed to solve problems and master the content.
Passages explaining important problem-solving strategies – content the student must learn – are clearly labeled with a LEARN THE STRATEGY label. Follow-up problems that require students to apply the just learned strategy are labeled with a USE THE STRATEGY label.
These labels, which are implemented throughout the entire text, allow students to easily find important content and practice its use. NEW and UPDATED! New and restructured features give students additional conceptual and skill building support.
UPDATED! Tutorial spreads and Design a Synthesis sections explicitly highlight essential skills. Marginal notes highlighting relative reactivity and the Organizing What We Know feature now align with each other to better relate skill building to conceptual understanding.
Newly highlighted Problem-Solving Strategies guide students on how to approach various problems and help to develop critical thinking skills. See more at:. Seven special Design A Synthesis sections introduce and help students through the iterative process of solving complex problems. End of chapter Problems review key topics and reinforce essential skills. End of chapter Reaction Summaries ensure students fully grasp how each reaction occurs. NEW and UPDATED! Applications boxes connect the discussion to Medical, Environmental, Biological, Pharmaceutical, Nutritional, Chemical, Industrial, Historical, and General applications, and help students relate the material to real life and potential future careers.
Bruice provides more of this kind of material than any other competing text on the market. Redesigned Organizing What We Know about the Reactions of Organic Compounds feature.
This informative and powerful table summarizes Bruice’s approach to teaching organic chemistry by categorizing all organic reactions into four groups having similar chemical behavior. Group I: electrophilic addition reactions. Group II: nucleophilic substitution reactions and elimination reactions.
Group III: nucleophilic ddition reactions and nucleophilic addition–elimination reactions. Group IV: electrophilic (and nucleophilic) aromatic substitution reactions. The table builds as students gain knowledge about groups of organic compounds as they work through the text. The easy to interpret table emphasizes the key characteristics common to the group of organic compounds covered in preceding chapters. Content Updates and Revisions to the Table of Contents streamline and improve clarity in the presentation. Using the E,Z system to name alkenes was moved to Chapter 4, so it appears immediately after using cis, trans to distinguish alkene stereoisomers. Catalytic hydrogenation and relative stabilities of alkenes was moved from Chapter 6 to Chapter 5 (thermodynamics), so it can be used to illustrate how H° values can be used to determine relative stabilities.
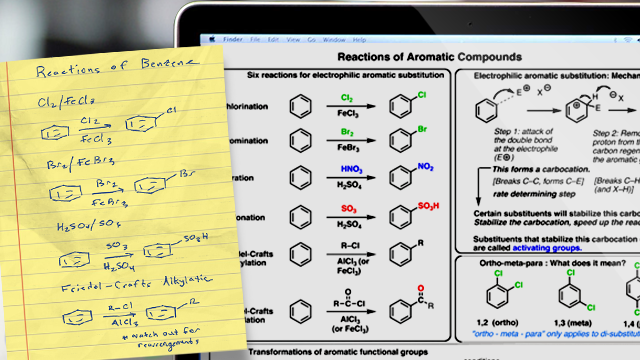
As a result, all the reactions in Chapter 6 have well-defined mechanisms where the first step in each of them is addition of the electrophile to the sp 2 carbon bonded to the most hydrogens. The two chapters in the previous edition on Substitution and Elimination reactions of alkenes have been combined into one chapter (Chapter 9). The recent compelling evidence showing that alkyl halides do not undergo S N1 solvolysis reactions has allowed this material to be greatly simplified, so now it fits nicely into one chapter. The structure of benzene is covered in Chapter 8 (the chapter on resonance) because it is the ideal compound to use to explain the concept of delocalized electrons.
A discussion on aromaticity is also found in this chapter, so a short introduction to electrophilic aromatic substitution reactions is now included. This allows students to see how aromaticity causes benzene to undergo electrophilic substitution rather than electrophilic addition—the reactions they have just finished studying. Electronic effects are discussed in Chapter 8 and used to teach students how substituents affect the p K a values of phenols, benzoic acids, and anilinium ions. Electronic effects are then reviewed in the chapter on benzene. Electronic effects can help students understand the directing effects of substituents on benzene rings. With the Eighth Edition, electronic effects are discussed in Chapter 8 in order to teach students how substituents affect the p K a values of phenols, benzoic acids, and anilinium ions.
Electronic effects are then reviewed in the chapter on benzene. Chapter on Lipids. The lipid material previously in the chapter on carboxylic acids and their derivatives (fatty acids, waxes, phospholipids, etc.) has been moved into a new chapter to help students see that the outcome of all reactions in the chapter could be determined simply by understanding how a tetrahedral intermediate partitions. The discussion of terpenes from the metabolism chapter has also been moved into this chapter, along with some new material. The IR spectra have been updated throughout the text and the solutions manual while additional spectroscopy problems have been added to the solutions manual.
Bruice’s Study Guide and Solutions Manual now offers a diverse group of 60 spectroscopy problems. New To This Edition Personalize learning with MasteringChemistry™. MasteringChemistry from Pearson is the leading online homework, tutorial, and assessment system, designed to improve results by engaging students before, during, and after class with powerful content. Instructors ensure students arrive ready to learn by assigning educationally effective content before class, and encourage critical thinking and retention with in-class resources such as Learning Catalytics ™. Students can further master concepts after class through traditional and adaptive homework assignments that provide hints and answer-specific feedback.
The Mastering gradebook records scores for all automatically graded assignments in one place, while diagnostic tools give instructors access to rich data to assess student understanding and misconceptions. Mastering brings learning full circle by continuously adapting to each student and making learning more personal than ever—before, during, and after class. Before Class. Organic Chemistry Dynamic Study Modules focus on general chemistry remediation, acid-base chemistry, functional groups, nomenclature, and key mechanisms. The modules help students study effectively on their own by continuously assessing their activity and performance in real time.
Here's how it works: students answer a set of questions and indicate their confidence level for each answer. The questions repeat until students answer them all correctly and confidently. Once completed, the module explains the key concept.
Dynamic Study Modules are available as graded assignments for prior to class, and are accessible on smartphones, tablets, and computers. Pearson eText 2.0 features include:. Available on smartphones and tablets. Seamlessly integrated videos and other rich media.
Accessible (screen-reader ready). Configurable reading settings, including resizable type and night reading mode. Instructor and student note taking, highlighting, bookmarking, and search During Class.
Learning Catalytics ™generates class discussion, guides your lecture, and promotes peer-to-peer learning with real-time analytics. MasteringChemistry with eText now provides Learning Catalytics—an interactive student response tool that uses students’ smartphones, tablets, or laptops to engage them in more sophisticated tasks and thinking. Instructors can:. Pose a variety of open-ended questions that help students develop critical thinking skills around structure and reactivity in organic chemistry with 10 new Learning Catalytics questions for each chapter of Organic Chemistry, Eighth Edition. Monitor responses to find out where students are struggling. Use real-time data to adjust the instructional strategy and engage students during class.
Manage student interactions by automatically grouping students for discussion, teamwork, and peer-to-peer learning After Class. MasteringChemistry's Organic Chemistry Drawing Tool is a customized version of Java Free MarvinSketch that accommodates the diversity of structures and reaction mechanisms inherent to learning organic chemistry while providing students with a wrong answer specific feedback.
Mastering Organic Chemistry Study Guide
This educational version of MarvinSketch has been customized in response to input from hundreds of undergraduate students. The drawing tool includes comprehensive MasteringChemistry tutorials, specific to drawing with MarvinSketch, that equip students to start quickly drawing organic structures and mechanisms to complete homework.
The tutorials cover how to accurately draw reaction mechanisms, how to modify answers, and how to use the palette. All mechanism-based problems provide feedback specific to each step of the reaction, and new visual cues help clarify exact placement of arrows, enable selection of the electron, and highlight which bonds have been formed or broken. Table of Contents PART ONE: An Introduction to the Study of Organic Chemistry 1. Remembering General Chemistry: Electronic Structure and Bonding 2. Acids and Bases: Central to Understanding Organic Chemistry TUTORIAL: Acids and Bases 3. An Introduction to Organic Compounds: Nomenclature, Physical Properties, and Structure PART TWO: Electrophilic Addition Reactions, Stereochemistry, and Electron Delocalization TUTORIAL: Using Molecular Models 4. Isomers: The Arrangement of Atoms in Space TUTORIAL: Interconverting Structural Representations 5.
Alkenes: Structure, Nomenclature, and an Introduction to Reactivity. Thermodynamics and Kinetics TUTORIAL: Drawing Curved Arrows 6. The Reactions of Alkenes. The Stereochemistry of Addition Reactions 7.
The Reactions of Alkynes. An Introduction to Multistep Synthesis 8. Delocalized Electrons: Their Effect on Stability, pKa, and the Products of a Reaction. Aromaticity and Electronic Effects: An Introduction the Reactions of Benzene TUTORIAL: Drawing Resonance Contributors PART THREE: Substitution and Elimination Reactions 9.
Substitution and Elimination Reactions of Alkyl Halides 10. Reactions of Alcohols, Ethers, Epoxides, Amines, and Sulfur-Containing Compounds 11. Organometallic Compounds 12. Radicals TUTORIAL: Drawing Curved Arrows in Radical Systems PART FOUR: Identification of Organic Compounds 13. Mass Spectrometry; Infrared Spectroscopy; and UV/Vis Spectroscopy 14. NMR Spectroscopy PART FIVE: Carbonyl Compounds 15.
Reactions of Carboxylic Acids and Carboxylic Acid Derivatives 16. Reactions of Aldehydes and Ketones. More Reactions of Carboxylic Acid Derivatives 17. Reactions at the α-Carbon TUTORIAL: Synthesis and Retrosynthetic Analysis PART SIX: Aromatic Compounds 18. Reactions of Benzene And Substituted Benzenes 19. More About Amines. Reactions of Heterocyclic Compounds PART SEVEN: Bioorganic Compounds 20.
The Organic Chemistry Of Carbohydrates 21. Amino Acids, Peptides, and Proteins 22. Catalysis in Organic Reactions and in Enzymatic Reactions 23. The Organic Chemistry of the Coenzymes, Compounds Derived from Vitamins 24. The Organic Chemistry of the Metabolic Pathways 25. The Organic Chemistry of Lipids 26.
The Chemistry of the Nucleic Acids PART EIGHT: Special Topics in Organic Chemistry 27. Synthetic Polymers 28. Pericyclic Reactions Appendix I p K a Values Appendix II Kinetics Appendix III Summary of Methods Used to Synthesize a Particular Functional Group Appendix IV Summary of Methods Employed to Form Carbon-Carbon Bonds Appendix V Spectroscopy Tables Appendix VI Physical Properties of Organic Compounds Appendix VII Answers to Selected Problems. About the Author(s) After graduating from the Girls’ Latin School in Boston, Paula Bruice earned an A.B. From Mount Holyoke College and a Ph.D. In chemistry from the University of Virginia. She then received an NIH postdoctoral fellowship for study in the Department of Biochemistry at the University of Virginia Medical School and held a postdoctoral appointment in the Department of Pharmacology at Yale Medical School.
Mastering Chemistry Student Access Kit Pearson
Paula has been a member of the faculty at the University of California, Santa Barbara since 1972, where she has received the Associated Students Teacher of the Year Award, the Academic Senate Distinguished Teaching Award, two Mortar Board Professor of the Year Awards, and the UCSB Alumni Association Teaching Award. Student Access Codes. Mastering Chemistry with Pearson eText - Standalone Access Card - for Organic Chemistry, 8/E Bruice ISBN-10:. ISBN-13: 111 ©2017.
Access Card Package, 1416 pp. URL (origin): Suggested retail price: $115.00 Net price: $0.00?.
Modified Mastering Chemistry with Pearson eText - Standalone Access Card - for Organic Chemistry, 8/E Bruice ISBN-10:. ISBN-13: 430 ©2017.
Access Card Package, 1416 pp. URL (origin): Suggested retail price: $115.00 Net price: $0.00?. Downloadable Instructor Resources. Instructor's Resource Materials (Download Only) for Organic Chemistry, 8/E Bruice ISBN-10:.
ISBN-13: 592 ©2017. Online. Live. (7.4MB) Clickerppts Chapters 01-28 available for download. (28.7MB) Worked Examples Chapters 01-28 available for download. (88.6MB) DigitalTransparencies-a Chapters 01-03 available for download. (77.2MB) DigitalTransparencies-b Chapters 04-07 available for download.
(86.2MB) DigitalTransparencies-c Chapters 08-10 available for download. (95.6MB) DigitalTransparencies-d Chapters 11-14 available for download. (91.3MB) DigitalTransparencies-e Chapters 15-18 available for download.
(130.3MB) DigitalTransparenciesf Chapters 19-23 available for download. (147.7MB) DigitalTransparenciesg Chapters 24-28 available for download.
(124.2MB) Image PPT a Chapters 01-04 available for download. (123.0MB) Image PPTb Chapters 05-08 available for download. (104.4MB) Image PPTc Chapters 09-12 available for download. (132.6MB) Image PPTd Chapters 13-15 available for download. (112.4MB) Image PPTe Chapters 16-19 available for download.
The following topics are fair game for the CHEM 1220 Exam #3 for Spring 2013. Under each topic there are practice problems from the text to help you study for the exam. These questions can also be found on Mastering Chemistry in teh Exam #3 Practice Problems assignment. The sheet attached to the exam is the same document attached to last exam.
Section 19.1 Spontaneous Processes Be able to distinguish between a spontaneous process, reversible process, irreversible process, and isothermal process. Section 19.2 Entropy and the Second Law of Thermodynamics State the second law of thermodynamics and describe the notion of entropy. Be able to justify how/why the entropy of the universe increases in a spontaneous process. Section 19.3 Molecular Interpretation of Entropy Explain how the entropy of a system is related to the number of possible microstates and describe the kinds of molecular motion that a molecule can possess. Be able to use the concepts of microstates and molecular motion to make qualitative predictions about entropy.
Section 19.4 Entropy Changes in Chemical Reactions Predict the sign of the entropy change for physical and chemical processes. 19.3, 19.4, 19.43, 19.47, 19.48, 19.49 Section 19.5 Gibbs Free Energy Be able to calculate changes in entropy, or changes in Gibbs free energy, for chemical reactions to determine if a reaction is spontaneous.
19.53, 19.54, 19.59, 19.61 Section 19.6 Free Energy and Temperature Recognize how changing the temperature influences the spontaneity of a reaction and predict which temperatures a reaction will be spontaneous given the change in enthalpy and the change in entropy. 19.66, 19.67, 19.69, 19.71 Section 19.7 Free Energy and Temperature Be able to calculate ΔG under non-standard conditions and recognize how ΔG changes when the partial pressures of gases are not equal to one atmosphere and the concentration of solutions are not one molar. Relate Gibbs free energy to the equilibrium constant and understand how the magnitude of K relates to spontaneity.
19.77, 19.81, 19.99 Understand the molecular interpretation of entropy, and how it relates to the 2nd law of thermodynamics. 19.27, 19.37, 19.43 Laboratory experiment #22 Solubility and Determination of ΔG, ΔH and ΔS of Ca(OH) 2 Understand calculations & concepts. Section 20.1 Oxidation States and Oxidation-Reduction Reactions Be able to determine the oxidation states of all species in an electrochemical reaction in order to identify the species oxidized, the species reduced, the oxidizing agent, and the reducing agent based on standard reduction potential data or reactivity. 20.6, 20.43, 20.47 Section 20.2 Balancing Redox Reactions Complete and balance redox equations using the method of half-reactions in both acidic and basic solutions. 20.21, 20.23 Section 20.3 Voltaic Cells Sketch a voltaic cell and identify its cathode, anode, and the directions that electrons and ions move. Be able to visualize the change in mass at the anode/cathode and the change in concentrations in the anode/cathode compartment at the molecular level.
20.8, 20.27, 20.37, 20.39 Section 20.4 Cell Potentials Under Standard Conditions Calculate standard cell potentials (emfs) from standard reduction potentials. Use reduction potentials to predict whether a redox reaction is spontaneous and to determine the relative strengths of oxidizing and reducing agents. Understand the terms standard reduction potential and the operation of a standard hydrogen electrode. 20.33, 20.34 Section 20.5 Free Energy and Redox Reactions Related the standard cell potential to the standard free energy change and the equilibrium constant. Section 20.6 Cell Potentials Under Nonstandard Conditions be able to apply the Nernst equation to determine the cell potential under non-standard conditions and relate the standard cell potential to the standard free energy change and the equilibrium constant. 20.65, 20.69, 20.72 Section 20.6 Cell Potentials Under Nonstandard Conditions Electrolysis in non-aqueous and aqueous systems. 20.89, 20.90, 20.109 Section 20.8 Corrosion Be able to apply concepts of spontaneous electrochemical processes to explain how corrosion is prevented by cathodic protection.
20.84, 20.85 Section 20.9 Electrolysis Describe the reactions in electrolytic cells and relate amounts of products and reactants in redox reactions to electrical charge. Laboratory experiment #23 Electrochemistry Understand calculations & concepts. Section 23.1-23.2 Transition Metal Complexes Determine the oxidation number and number of d electrons for metal ions in complexes and determine the coordination number about the central atom and geometry of the transition metal complex.
23.15, 23.23, 23.25, 23.29 Section 23.4 Isomerization in Coordination Chemistry Recognize and draw the structural and stereoisomers in a transition metal complex. 23.5, 23.6, 23.39, 23.40 Section 23.4 Isomerization in Coordination Chemistry Section 23.6 Crystal-Field Theory Be able to interpret crystal-field splitting diagrams for octahedral, square-planar & tetrahedral geometries, and for weak and strong field ligands. Understand the terms high spin and low spin. 23.8, 23.47, 23.59, 23.63. Use crystal-field theory to explain color in coordination compounds. 23.51, 23.53, 23.54.